Highthroughput study of anisolvents on the stability of MHPs through robotics and ML Approaches.Pdf
() - Kate Higgins1, Maxim. Ziatdinov2,3, Sergei V. Kalinin2 and Mahshid Ahmadi1
- Link:
- DOI:
- Zotero Link: Highthroughput study of anisolvents on the stability of MHPs through robotics and ML Approaches.Pdf
- Tags: #paper
- Cite Key: [@HighthroughputStudyAnisolvents]
- Linked notes: Paper Annotations
Abstract
Notes
Annotations (9/5/2022, 11:39:38 AM)
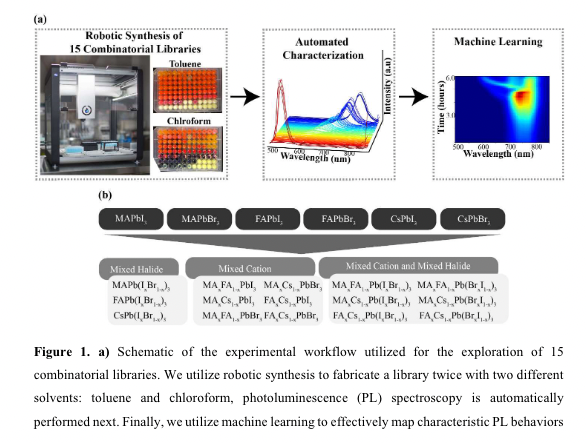
“Different combinations of the endmembers, MAPbI3, MAPbBr3, FAPbI3, FAPbBr3, CsPbI3, and CsPbBr3, are used to synthesize 15 combinatorial libraries, each with 96 unique combinations. In total, roughly 1100 different compositions are synthesized. Each library is fabricated twice using two different antisolvents: toluene and chloroform. Once synthesized, photoluminescence spectroscopy is automatically performed every 5 minutes for approximately 6 hours.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 1)
“Different combinations of the endmembers, MAPbI3, MAPbBr3, FAPbI3, FAPbBr3, CsPbI3, and CsPbBr3, are used to synthesize 15 combinatorial libraries, each with 96 unique combinations. In total, roughly 1100 different compositions are synthesized. Each library is fabricated twice using two different antisolvents: toluene and chloroform. Once synthesized, photoluminescence spectroscopy is automatically performed every 5 minutes for approximately 6 hours” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 1)
(“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 1) Antisolvant : toluent and chloroform
“Non-negative Matrix Factorization (NMF) is then utilized to map the time- and compositional-dependent optoelectronic properties. T” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 1)
“A multitude of studies have demonstrated how the incorporation of other cations, particularly cesium (Cs+)15 and formamidinium (FA+)16 into methylammonium (MA) systems, leads to improve stability in ambient and operational conditions” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 3)
“multitude of studies have demonstrated how the incorporation of other cations, particularly cesium (Cs+)15 and formamidinium (FA+)16 into methylammonium (MA) systems, leads to improve stability in ambien” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 3)
“ch requires the rapid application of the antisolvent to a perovskite solution, and the antisolvent then extracts the solvent, leading to the fast supersaturation of the perovskite precursor and subsequent precipitation.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 3)
“Wang et al. postulated that the use of chlorobenzene mixed with iso-propyl alcohol removes residual chlorobenzene, therefore, increasing the grain size of the perovskite film and drop in defect density22 .” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 3)
“chlorobenzene mixed with iso-propyl alcohol removes residual chlorobenzene” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 3)
“he grain size of the perovskite film and drop in defect density” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 3)
“Gu et al. utilized a robot-based high-throughput workflow to screen antisolvents on single component MHPs24. By testing 48 different organic ligands as an antisolvent and three commonly used solvents: dimethyl sulfoxide (DMSO), γ-butyrolactone (GBL), and N'N-dimethylformamide (DMF), they explore how the choice of solvent and antisolvent effect on the formation of microcrystals” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 4)
“g 48 different organic ligands as an antisolvent and three commonly used solvents: dimethyl sulfoxide (DMSO), γ-butyrolactone (GBL), and N'N-dimethylformamide (DMF), they explore how the choice of solvent and antisolvent effect on the formation of microcrysta” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 4)
“Here, we utilize our previously reported workflow27” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 4)
“we synthesize 15 different binary perovskite systems, also referred to as a combinatorial library, twice utilizing two different solvents: toluene and chloroform.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 4)
“synthesize 15 different binary perovskite systems, also referred to as a combinatorial library, twice utilizing two different solvents: toluene and chloroform.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 4)
“In each combinatorial library, there are 96 unique compositions, meaning that roughly 1100 unique compositions were synthesized. We then utilize an antisolvent approach to precipitate microcrystals4, 29.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 5)
“ombinatorial library, there are 96 unique compositions, meaning that roughly 1100 unique compositions were synthesized” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 5)
“Commonly used antisolvents documented by other works are classified into two categories: halogenated (chlorobenzene30 and chloroform29) and non- halogenated (toluene17, anisole31, diethyl ether32, and ethyl acetate33). Here, we chose to utilize two antisolvents: chloroform and toluene, as they represent each category and because these antisolvents are heavily used in the production of high-quality perovskites.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 5)
“We control the composition of each well by programming the robot to pipette the desired quantity of the endmember solution. After the deposition of the endmember solutions, the antisolvent is pipetted into each well. The formation of microcrystals is apparent by the color change that occurs immediately upon the addition of the antisolvent.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 6)
“control the composition of each well by programming the robot to pipette the desired quantity of the endmember solution.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 6)
“After the deposition of the endmember solutions, the antisolvent is pipetted into each well” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 6)
“mpositional dependent optoelectronic properties, we utilize an automated Multi-Mode well plate reader with the capabilities to perform photoluminescence (P” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 6)
“We can measure the PL spectra of 96 compositions in roughly 5 minutes.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 6)
“PL spectra of 96 compositions in roughly 5 minutes” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 6)
“To effectively map the time- and compositional-dependent PL properties and stability relationships concurrently, we adopt a multivariate statistical approach, in particular, Non-negative Matrix Factorization (NMF)27.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 6)
“ultivariate statistical approach, in particular, Non-negative Matrix Factorization (NM” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 6)
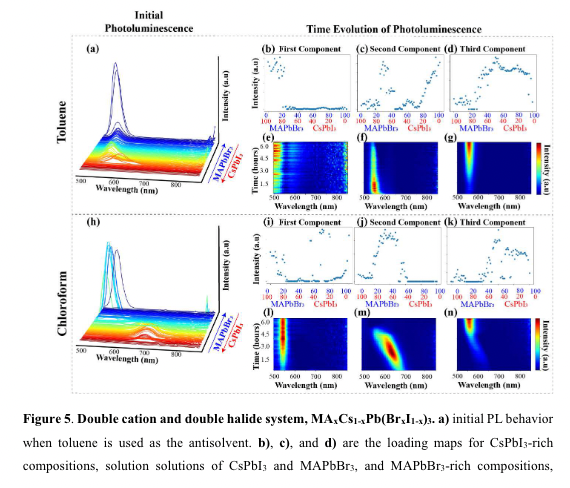
“While all combinatorial libraries
are fully explored in the Supporting Information, here, we focus on only 4 combinatorial
libraries: a mixed cation system, MAxFA1-xPbI3, a mixed halide system, MAPb(IxBr1-x)3, and two
mixed cation and mixed halide systems, MAxCs1-xPb(IxBr1-x)3 and MAxCs1-xPb(BrxI1-x)3.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 7) What does it mean?
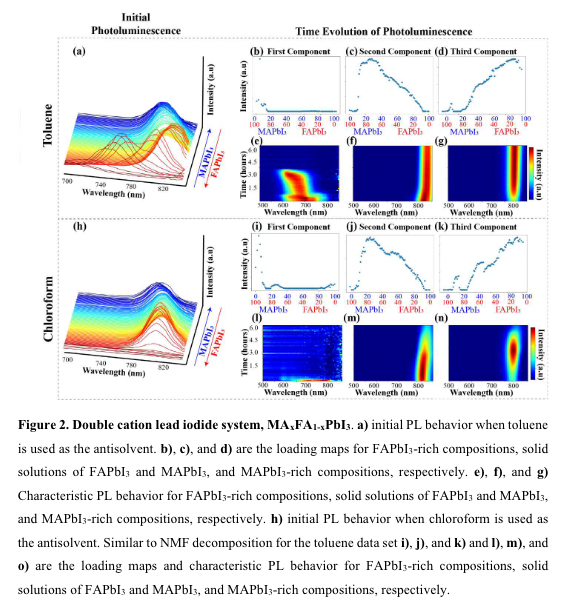
“otoinactive, yellow phase (δ-FAPbI3) has formed in combination with the black phase (α-FAPbI3)” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 9)
“we reach two conclusions: 1) neither toluene nor chloroform is the appropriate antisolvent choice for the formation of a pure phase α-FAPbI3, and 2) stabilization of the α-FAPbI3 phases can be achieved through small amount of MAPbI3 in agreement with previous studies40 .” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 9)
“neither toluene nor chloroform is the appropriate antisolvent choice for the formation of a pure phase α-FAPbI3, and 2) stabilization of the α-FAPbI3 phases can be achieved through small amount of MAPbI3 in agreement with previous studies4” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 9)
“by exploring the time-dependent PL behavior, we conclude this double cation system is vastly more stable when toluene is utilized as the antisolvent instead of chloroform.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 9)
“double cation system is vastly more stable when toluene is utilized as the antisolvent instead of chloro” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 9)
“better stability of these mixed phases in toluene rather than chloroform” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 9)
“tter stability of these mixed phases in toluene rathe” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 9)
“Other studies have confirmed that the stabilization of α-FAPbI3 in ambient conditions can be achieved through small amounts of doping with MAPbI340.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 9)
“the stabilization of α-FAPbI3 in ambient conditions can be achieved through small amounts of doping with MAPbI” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 9)
“possibly causing the sample to revert to PbI2 or forming a hydrate product41
.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 9) When reacting with moisture the sample revert to PbI2 or forming hydrates product
“sibly causing the sample to revert to PbI2 or forming a hydrate product41” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 9)
“Our conclusions that toluene is a better choice for this system is further confirmed by analyzing the time-dependent PL behavior of the MAPbI3-rich compositions.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 9)
“fter a small increase in PL intensity at the beginning, indicating possible filling of trap state” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 10)
“A similar increase in PL intensity is observed for the system when chloroform is used, as shown in Figures 2k) and 2n); however, after approximately 5 hours, the PL intensity decreases, indicating the formation of hydrate products is occurring for these samples as well41.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 10)
“We postulate that chloroform does not fully extract the GBL, and, therefore, leaves unreacted precursors to interact with the air, eventually causing degradation.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 10)
“We postulate that chloroform does not fully extract the GBL, and, therefore, leaves u” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 10)
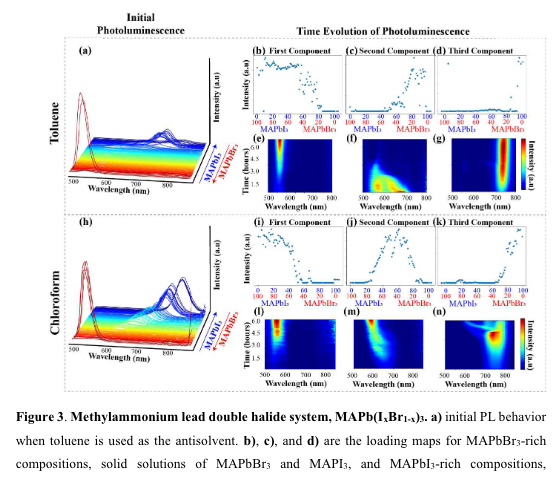
“MAPbI3-rich compositions have comparatively a large PL intensity as compared to the same compositions when toluene was used.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 11)
“r MAPbBr3-rich compositions, we observe an increase in PL intensity without any shift in peak position for both toluene” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 11)
“For example, as shown in Figures 3c) and 3f), a broad peak, likely indicative of two convolved peaks, is present initially. This peak begins to separate into two, one representing the bromine-rich phase and mixed phase. A” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 11)
“as shown in Figures 3c) and 3f), a broad peak, likely indicative of two convolved peaks, is present ini” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 11)
“Simultaneously, when these same compositions are synthesized using chloroform as the antisolvent, we observe the presence of a broad, low-intensity peak.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 11)
“road, low-intensity pe” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 11)
“.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 11)
“. In both cases, we observe halide segregation caused by reactions with the environment35, indicating this halide segregation cannot be prevented for these particular composition ranges” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 11)
“In both cases, we observe halide segregation caused by reactions with the environment35, indicating this halide segregation cannot be prevented for these particular composition ranges.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 11)
“APbI3-rich compositions when chloroform is used, we observe an increase and subsequent decrease in PL intensity as they are exposed to air” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 12)
“ither antisolvent produces a stable, well-defined PL peak for MAPbBr3-rich compositions; however, toluene is the more appropriate choice when incorporating small amounts of MAPbBr3 into MAPbI3.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 12)
“antisolvent produces a stable, well-defined PL peak for MAPbBr3-rich compositions; however, toluene is the more appropriate choice when incorporating small amounts of MAPbBr3 into MAPbI3.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 12)
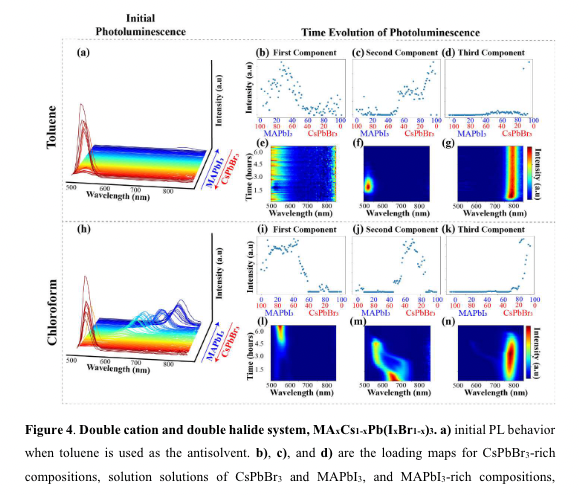
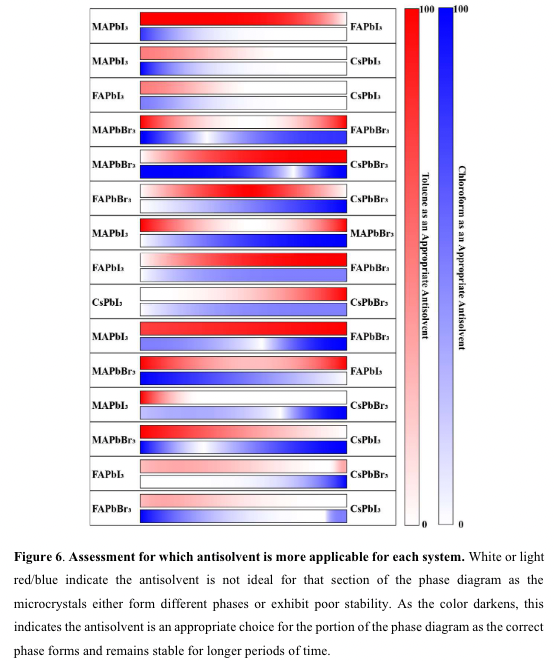
“toluene is a more beneficial antisolvent for MAPbI3 and chloroform is a better
antisolvent for CsPbBr3. A” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 13) Antisolvant choice:
toluent -> MAPbI3 rich
Chloroform -> CsPb
“oluene is a more beneficial antisolvent for MAPbI3 and chloroform is a better antisolvent for CsPbBr3” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 13)
“when chloroform is used, we initially observe a mixed-phase PL peak, as shown in Figure 4j) and 4f); however, after approximately 2 hours, this peak separates into two peaks, each representing the iodide-rich and bromide-rich phases, confirming halide segregation35 .” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 13)
“en chloroform is used, we initially observe a mixed-phase PL peak, as shown in Figure 4j) and 4f); however, after approximately 2 hours, this peak separates into two peaks, each representing the iodide-rich and bromide-rich phases, confirming halide segregation3” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 13)
“we were surprised by the finding that toluene was not the ideal antisolvent for solid solutions between CsPbBr3 and MAPbI3, considering there have been studies showing how toluene produces high-quality thin films when a combination of DMSO and GBL is used17 .” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 14)
“toluene was not the ideal antisolvent for solid solutions between CsPbBr3 and MAPbI3,” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 14)
“n of this phase is not dependent on the antisolvent, as evidence by its presence when chloroform is used, as shown in” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 15)
“tead, this phase is introduced by exposure to ambient conditi” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 15)
“For both systems, it is demonstrated that toluene is not the appropriate choice for solid solutions of CsPbX3- rich and methylammonium lead halide.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 16)
“r both systems, it is demonstrated that toluene is not the appropriate choice for solid solutions of CsPbX3rich and methylammonium lead hali” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 16)
“Conversely, in both systems, chloroform produces initial mixed phase compositions; however, eventually, halide segregation begins to occur. Overall, this comparison is indicative of the precursor having an effect of the types of dynamical processes to occur as caused by the solvent in which the endmember is dissolved.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 16)
“Conversely, in both systems, chloroform produces initial mixed phase compositions; however, eventually, halide segregation begins to occur” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 16)
“n 15 different binary systems, analyzing approximately 1100 unique compositio” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 18)
“We have shown remarkable stability in ambient conditions for microcrystals of MAPbI3- rich compositions and solid solutions between FAPbI3 rich and MAPbI3 compositions when toluene is utilized.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 18)
“icrocrystals of MAPbI3rich compositions and solid solutions between FAPbI3 rich and MAPbI3 compositions when toluene is utili” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 18)
“all together the absolute synthesis and characterization time total only 1.5 weeks” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 18)
“all together the absolute synthesis and characterization time total only 1.5 weeks.” (“highthroughput study of anisolvents on the stability of MHPs through robotics and ML approaches.pdf”, p. 18)
Given by Nripan on 16/8/2022